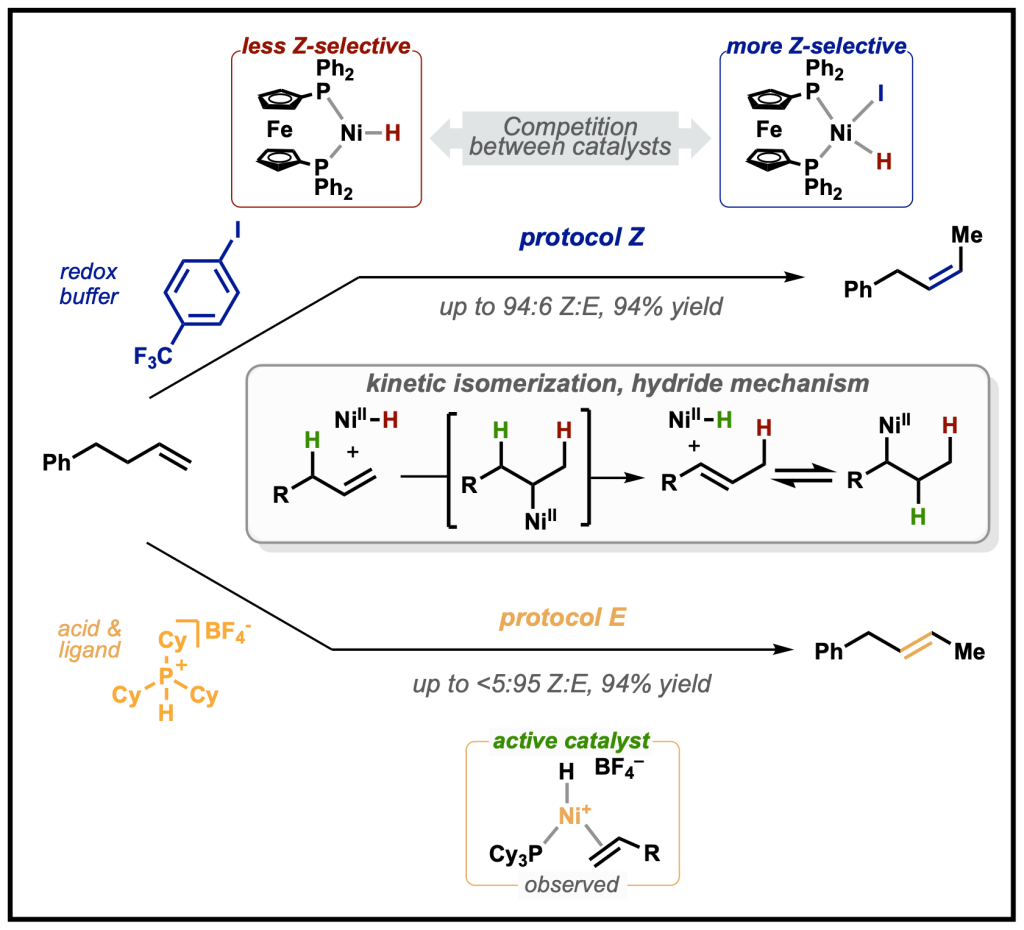
The final peer-reviewed version of our nickel-catalyzed stereodivergent olefin isomerization paper appears online today in Angewandte Chemie. High Z-selectivity is facilitated by the combination of a bisphosphine ligand and an electron-deficient aryl iodide additive, which serves as a redox buffer to ensure the nickel catalyst is held in the +2 oxidation state. On the other hand, high E-selectivity is enabled by the use of a bulky trialkylphosphonium salt, which functions as the ligand and initial hydride source. Congrats to all of the authors, Camille, Anne, Calvin, Shenghua, and Zi-Qi, and thanks to our fantastic collaborator Julien of Syngenta!
For a link to the paper in Angew. Chem. Int. Ed., click here: https://onlinelibrary.wiley.com/doi/10.1002/anie.202320081
As a reminder, a ChemRxiv pre-print on this work was uploaded in December 2023: https://chemrxiv.org/engage/chemrxiv/article-details/658b9e9766c13817291f94af